Abstract
The techniques employed to mobilise autologous peripheral blood stem cells in patients with myeloma have evolved over time and vary between countries. The most common methods are (1) Cyclophosphamide and Granulocyte - Colony Stimulating Factor (G-CSF) (Cyclophosphamide), (2) G-CSF only, (3) G-CSF and Plerixafor, and (4) Other. In general, chemotherapy, usually Cyclophosphamide, is used in Europe and single agent G-CSF in North America.
We performed a retrospective analysis of the EBMT database to analyse stem cell mobilisation techniques in European patients undergoing a first ASCT for myeloma over the last decade. This data was available on a select cohort of patients for whom Med B form data had been submitted.
A total of 76,923 patients received a first autologous stem cell transplant for myeloma in 501 EBMT-affiliated centres between 2012 and 2021. Detailed Stem cell mobilization data including CD34 results were available on 4,710 (M 58%: F 42%) patients from 87 centers. The Median (IQR) age was 61.3 (54.7-66.1) years. MM subtypes were IgG, IgA, Light Chain and Other in 56.6%, 19.2%, 20.8% and 3.4%, respectively. The stage at first transplant was CR, VGPR/PR, SD/MR, and Rel/Prog in 17.5%, 78.4%, 2.8% and 1.3%, respectively.
The frequency of use of each of the stem cell mobilization techniques were as follows: Cyclophosphamide (n=3,017, 67.1%), G-CSF only (n=1,207, 26.9%), G-CSF + Plerixafor (n=222, 4.9%) and Other (47, 1.0%). Peripheral blood stem cells were the sole stem cell source in 4,705 (99.9%) of the transplants.
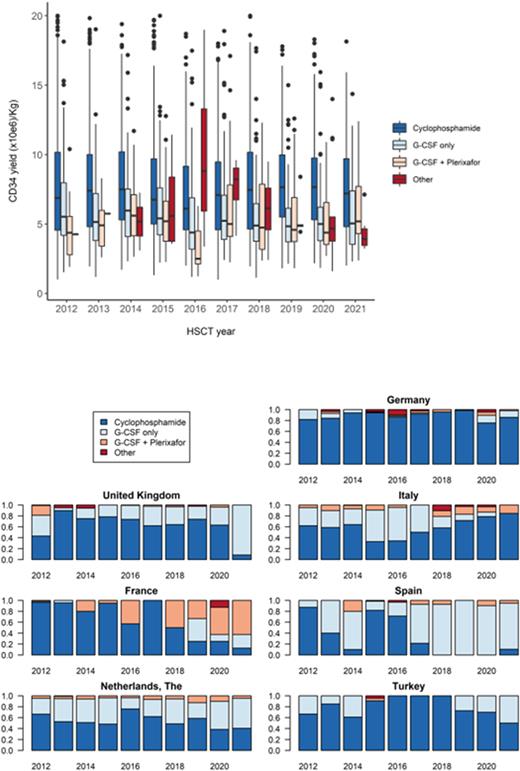
The comparative median annual CD34 yields (x10e6/kg) are shown below and reveal that the yields following Cyclophosphamide-based mobilization were consistently higher (6.14-7.8) than those following either G-CSF (4.46-6.1) or G-CSF and Plerixafor (2.5-5.6). When analyzed in aggregate, the median (IQR) stem cell yields (x10e6/kg) following Cyclophosphamide, G-CSF, G-CSF and Plerixafor, and Other were 7.32 (4.9-10.14), 5.25 (3.88-7.38), 4.94 (3.42-6.85) and 4.9 (3.82-7.54), respectively.
When analysed by MM subtype, there was no significant difference in yield between the IgG, IgA, Light Chain and Other categories (p=0.523).
We next analyzed the median annual CD34 yields (x10e6/kg) by induction regimen (no. of pts): CTD (Cyclophosphamide, Thalidomide, Dexamethasone) (n=712) 7.56, Other (n=623) 5.86, PAD (Bortezomib, Adriamycin, Dexamethasone) (n=256) 5.8, VAD (Vincristine, Adriamycin, Dexamethasone) (n=172) 5.03, VCD (Bortezomib, Cyclophosphamide, Dexamethasone) (n=1,735) 6.21, VD (Bortezomib, Dexamethasone) (n=603) 5.97, VRD (Bortezomib, Lenalidomide, Dexamethasone) (n=811) 5.42 and VTD (Bortezomib, Thalidomide, Dexamethasone) (n=3,192) 7.12 (p<0.001), the highest yields being collected following CTD and VTD (<0.001).
We finally examined mobilization trends in the seven largest countries in the dataset (France, Germany, Italy, the Netherlands, Spain, Turkey, and the United Kingdom) over time. Data was available for between 9.6% and 22.8% of patients in 6 of the 7 countries; however, it was only available in 2.9% of patients from Germany. Within these clear limitations, there were wide national variations in clinical practice both over time and between countries, as shown below.
Over the last decade, two-thirds of patients in EMBT centres underwent autologous stem cell collection following Cyclophosphamide and G-CSF, one quarter following single agent G-CSF and 5% following G-CSF and Plerixafor. As reported previously, yields were consistently higher following chemotherapy-based mobilization. However, we have no data on associated morbidity such as the incidence of infection, the need for hospital admission or costs. As regards the effect of prior induction regimens, the highest yields were seen in patients who had received either CTD or VTD induction, possibly reflecting less stem cell toxicity due to chemotherapy and/or deeper remissions following these regimens. Finally, limited data on trends in practice appears to show considerable variation between countries combined with significant changes in some countries over the last decade.
Disclosures
Hayden:Amgen: Other: Participation in Advisory Board. Snowden:Novartis: Speakers Bureau; Mallinckrodt: Speakers Bureau; Gilead: Speakers Bureau; Janssen and Jazz: Speakers Bureau; Medac: Membership on an entity's Board of Directors or advisory committees; Kiadis: Other: clinical trial IDMC membership . Griskevicius:Miltenyi Biomedicine: Membership on an entity's Board of Directors or advisory committees. Drozd-Sokolowska:Servier: Honoraria, Speakers Bureau; AbbVie: Honoraria, Speakers Bureau; Janssen: Honoraria; Roche: Consultancy; Sanofi: Honoraria. Beksac:Oncopeptides: Consultancy, Honoraria, Other: Advisory Boards, Speakers Bureau; Amgen: Consultancy, Honoraria, Other: Advisory Boards, Speakers Bureau; Sanofi: Consultancy, Honoraria, Other: Advisory Boards, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Advisory Boards, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: Advisory Boards, Speakers Bureau. Schönland:Pfizer: Honoraria; Takeda: Honoraria, Other: Travel Support; Janssen: Honoraria, Other: travel support, Research Funding; Prothena: Honoraria, Other: Travel Support, Research Funding. McLornan:CELGENE BMS: Research Funding, Speakers Bureau; ABBVIE: Speakers Bureau; NOVARTIS: Honoraria, Research Funding, Speakers Bureau; JAZZ: Honoraria, Speakers Bureau. Yakoub-Agha:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Janssen: Honoraria; Bristol Myers Squibb: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal